Overview
Cancer is a major public health concern in India. Cancer is enormously complex and highly adaptable; many subtypes of the disease have distinct clinical features and susceptibilities to therapy. Many cancers are still not diagnosed until they are at advanced stages and some resist most attempts at treatment. With advancement in scientific tools and technology, the mystery of cancer is unraveling and the Department of Biotechnology continues to support and promote basic, translational and inter-disciplinary research through a dedicated Technical Expert Committee on Cancer Research Biology. The present thrust largely is on:
- Cancer Diagnostics: With advancements in cancer genomics and improved computational capabilities in the country, the Department of Biotechnology through its Cancer Disease Biology Program, is dedicated to push the boundaries of early cancer detection. The program aims on advancing research to develop next-generation, minimally invasive techniques to identify cancer at its earliest stages. By leveraging the power of artificial intelligence (AI) and machine learning (ML), the program aims to enhance the analysis of complex data, enabling more precise diagnoses and prognostic options for cancer patients.
- Cancer Therapeutics: In addition to diagnostics, the program actively promotes the development of novel, affordable and accessible therapies, including cutting-edge CAR-T (Chimeric Antigen Receptor T-cell) therapies, Oncolytic viruses-based and phage based therapies, RNA therapeutics etc. With advancements in molecular biology, onco-immunology and better understanding of tumor biology, these therapies are expected to evolve and may soon become a cornerstone in cancer treatment. CAR-T therapy that has shown significant promise in treating certain cancers, particularly hematologic malignancies will be expanded for a broader spectrum of cancers, including solid cancers.
- Cancer Biology: Significant efforts are being made in advancing cancer research and deepening our understanding of the disease’s mechanisms and progression including metabolic processes that drive cancer growth and opportunities to target unique pathways for therapeutic intervention. Furthermore, the program aims on improving holistic care for cancer survivors, addressing challenges and research gaps during chemo/radio therapy and post-chemotherapy.
The ultimate goal is to foster cancer research and transform cancer care towards proactive healthcare, by ensuring earlier detection, more personalized treatments, and better support for survivors.
Major Programs & Initiatives
The support during the past few years has been particularly dynamic, with a series of exciting findings and transformative discoveries emerging from numerous projects supported across India. These findings not only enhance our understanding of cancer biology but also pave the way for novel diagnostic tools, therapeutic strategies, and more accessible cancer care solutions. By fostering collaboration and driving research in both basic and clinical domains, the program has been able to bridge the gap between scientific discovery and real-world application, ultimately striving to improve patient outcomes.
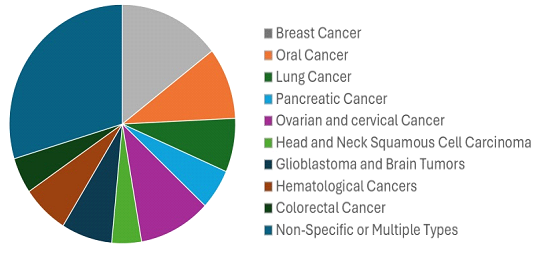
Figure 1 : Funding across cancer types in last five years
Exploring New Frontiers and Strengthening Indigenous Efforts for early translation:
Some of the early translational leads created in the past five years are now being advanced into products aimed at human interventions. One such example is a small molecule chemical inhibitor – ‘Disarib’ that targets BCL2, an antiapoptotic protein which is over expressed in various cancers. The first phase of pre-clinical toxicological studies in mice & rat has been completed, and results demonstrate high efficacy and reduced toxicity. Further this is being evaluated for the repeated dose toxicity studies in a non-rodent model at GLP certified laboratory, which if successful, will help in taking forward to Phase I clinical trial. This study has resulted in a patent filed for the combinatorial potential of Disarib, the BCL2 inhibitor.
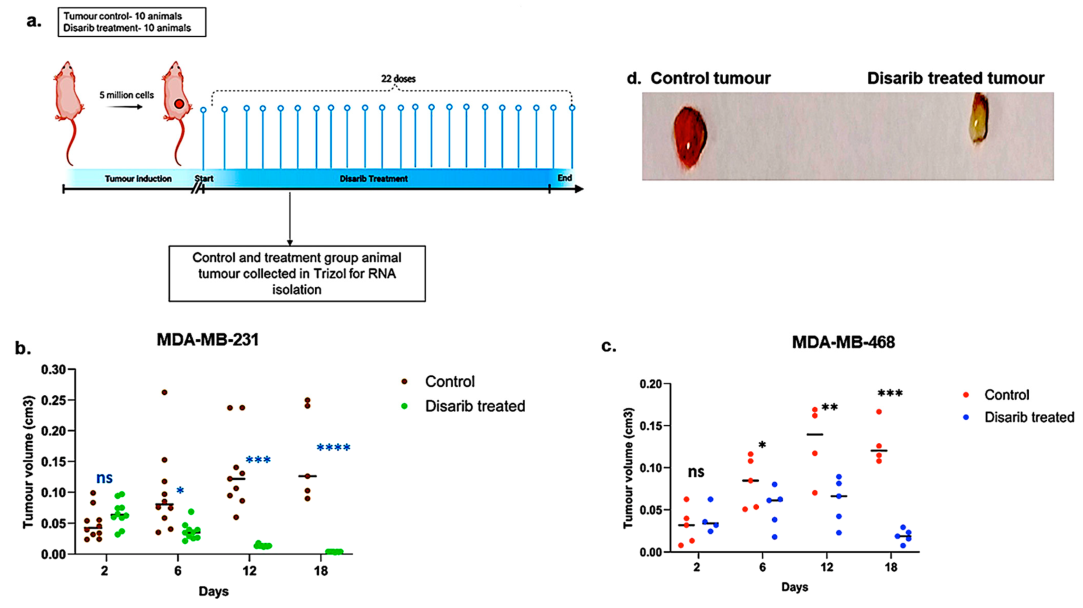
Figure 2 : Effect of Disarib on tumor proliferation in cell line-derived xenografts.
- Methodology opted for the experiment. Animals were treated with Disarib (50 mg/kg, 22 doses, daily, n = 10), and tumour regression was compared with the control group.
- Graph showing tumour regression in MDA-MB-231-injected nude mice after Disarib treatment.
- Graph showing tumour regression in MDA-MB-468-injected nude mice after Disarib treatment. Tumour volume is measured in cm3 for control and treated animals as plotted. where ns: non-significant p
- ) Representative image of a cell line-derived tumour control and a tumour treated with Disarib.
Another promising lead is a Chimeric Interleukin-15 (IL-15) developed using tools of genetic engineering. IL-15 is a cytokine which is a multifunctional cytokine that targets many cell types and connects the innate with the adaptive immune system. Globally, among the cytokines, IL-15 has been identified as a top candidate for cancer immunotherapy. However, the major limitations in developing IL-15 as a therapeutic agent are its short-half life and poor bioavailability. To overcome these limitations, stable chimeric IL-15 (IL-15 coupled to IgG2 constant heavy chain) was developed with increased half-life of 40 hr. However, despite the development of a stable chimeric IL-15 with extended half-life and bioactivity, its full therapeutic potential remains to be explored. Current studies are in progress to elucidate 3D structure &to investigate its role in combination therapy with checkpoint inhibitors for tumor regression and relapse prevention, as well as its potential as an adjuvant for formation of memory T and B cells. An Indian patent has been granted in 2024 (Patent: No. 529118)
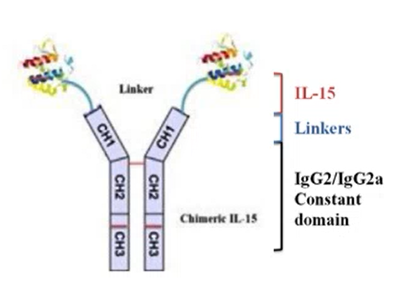 | 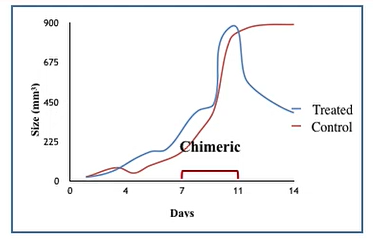 |
Figure 3 : A. Chimeric IL-15 B. Tumor regression after ChIL-15 treatment
Overcoming chemoresistance remains a major challenge in cancer therapy where cancer cells develop resistance to DNA-damaging agents by enhancing their DNA repair capacity, particularly through homologous recombination (HR).
The BLM protein plays a critical role in DNA damage sensing and repair by interacting with key DNA repair proteins, including RAD54, a chromatin remodeler. Dysfunctional BLM is associated with Bloom syndrome (BS), a rare genetic disorder that predisposes patients to a broad spectrum of cancers, including solid tumors, leukemias, and lymphomas. Paradoxically, in several cancers, such as colon cancer, BLM is aberrantly overexpressed, which is correlated with poor patient outcomes. In one of the studies researchers identified a specific 32-amino acid stretch of BLM (181-212aa) that efficiently stimulates RAD54's chromatin remodeling activity, leading to reduced DNA damage levels and increased chemoresistance to agents like camptothecin, cisplatin, and oxaliplatin.
For targeting this interaction to develop a therapeutic intervention FDA-approved Prestwick small-molecule library comprising 1277 compounds was screened. This effort resulted in the identification and validation of three small molecules that disrupt the RAD54-BLM interaction. In mouse models, combining these small molecules with camptothecin or oxaliplatin significantly suppressed chemoresistanttumor growth in a BLM-dependent manner.
Thus, these small molecules are promising candidates for adjunct therapy in colon cancer when combined with existing chemotherapeutic agents. This study was published in Journal of Clinical Investigation in Feb,2024. (https://www.jci.org/articles/view/161941#SEC3). The next step involves evaluating the efficacy of these drugs in colon cancer patients and currently, these are in phase I clinical trials.
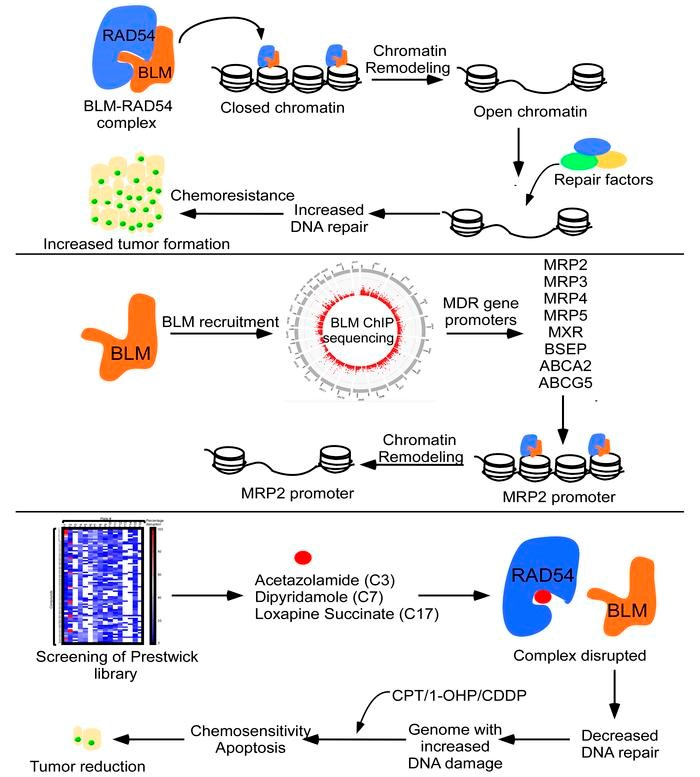
Figure 4: Identification &Characterization of Small molecules that disruptRAD54-BLM interaction resulting in tumor reduction colon cancer chemoresistance models
In a recently concluded project a DNA aptamer was developed that can bind RB cells and primary tumours with high specificity and affinity. The aptamer can be conjugated with therapeutic agents, including small interfering RNAs (siRNAs), CRISPR-Cas9, and chemotherapeutic drugs to enhance therapeutic efficacy and reduce associated toxicities. Further studies are underway to identify the receptor of this aptamer and assess the effectiveness of the functionality of this aptamer for targeted delivery.
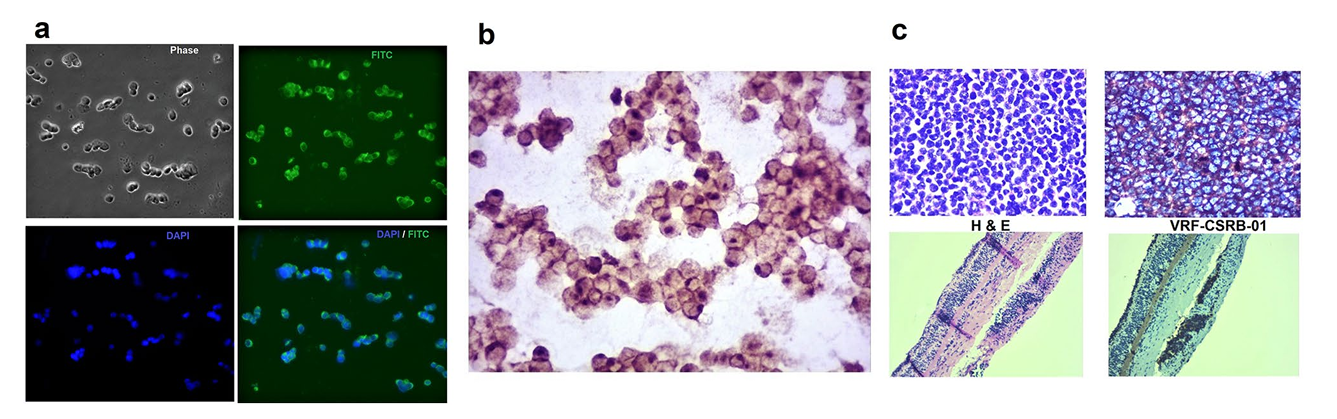
Figure 5 : The binding ability of VRF-CSRB-01 to Weri-RB1 by IFC, ICC and RB tumour sections by IHC. (a) Microscopy results showed membrane positivity of VRF-CSRB01 by the fluorescence imaging in Weri-RB1. (b) ICC of Weri-RB1 cells incubated with biotin labelled VRF-CSRB-01 and analysed by light microscope. (c) H and E staining and () IHC with biotin labelled VRF-CSRB-01 of primary retinoblastoma tumours and retina respectively.
Bridging Lab Discoveries to Patient Care:
A Collaborative Approach: PSMD10 (Gankyrin) is an oncoprotein overexpressed in several cancers. PSMD10, due to its role in influencing various hallmarks of cancer, is a promising target for cancer therapy. In a multi-institutional project, a team of researchers are now exploring ways for targeting the metastasis process of cancer, which accounts for more than 90% of cancer-related death, through targeting Gankyrin and its interacting partners, mainly CLIC1. Initial results of the study have revealed some interesting findings on the role of PSMD10 and CLIC1 in promoting cell migration in cancer cell lines. This is for the first time the nexus between a membrane channel protein (CLIC1) and a chaperone (Gankyrin) in cancer cell migration, and invasion is being explored using several tools in biophysics, biochemistry, cell biology, chemical biology, synthetic chemistry, structure-based inhibitor design, small molecule screening, and computational methods. This collaborative effort aims to bridge the gap between benchside research and bedside application, while also shortening the timeline for laboratory discoveries to reach the right patient cohorts.
Advancing Immunotherapy:
All existing CAR-T cell products available globally are autologous (made with same patient-derived T lymphocytes) to avoid severe alloimmune rejection due to a mismatch of MHC between the donor and the recipient. Various studies have been implemented through Department’s support to advance CAR-T cell therapy for a broader spectrum of cancers and reducing therapy related toxicities. One such study aims to develop ‘off-the shelf’ or ‘universal’ CAR-T cells from healthy donors using CRISPR/Cas9 technology with specific modifications in MHC genes. Concurrently, the project focused on designing and engineering "Inducible CARs" to mitigate cytokine release syndrome, a common adverse event associated with CAR-T cell therapy. Two constructs, CAR-1 and CAR-2, were designed, incorporating advanced features such as syn-NOTCH domains for context-specific activation and dual-plasmid systems enabling tight regulation of IL-6 secretion.
The Department has also supported several innovative projects aimed at advancing next-generation cancer immunotherapies. These initiatives include the development of dual CAR-T cells, NK cell-based treatments, cytokine-enhanced therapies such as IL-15 CAR-T, oncolytic virus-based therapies, and RNA therapeutics.
Harnessing Technology for Improved Diagnosis and Prognosis in Cancer:
Minimal Residual Disease Estimation in Multiple Myeloma using Image Processing: A Unit of Excellence supported at AIIMS, New Delhi led to the development of a computerized image analysis software for the classification of neoplastic plasma cells (PC) and normal PC for estimating minimal residual disease (MRD) in multiple myeloma (MM). The team with the help of machine learning methods developed a new risk stratification system – Modified Risk Staging (MRS). This AI-enabled risk-staging system - MRS (a risk calculator) is done for newly diagnosed multiple myeloma patients. It is an efficient and readily employable risk prognostication method that is beneficial for the settings where genomics tests cannot be performed owing to geographical or economical constraints. The findings have been published in Translational Oncology 2021 (https://doi.org/10.1016/j.tranon.2021.101157)
The team also have designed an innovative AI-based model, namely, the Bio-inspired Deep Learning architecture for the identification of altered Signaling Pathways (BDL-SP) to discover pivotal genomic biomarkers that can potentially distinguish Multiple Myeloma (MM) cancer from its precancerous stage i.e Monoclonal gammopathy of undetermined significance (MGUS). For this, whole exome sequencing data of 1174 MM and 61 MGUS patients were analyzed. In the quantitative benchmarking with the other popular machine learning models BDL-SP performed almost similar to the two other best performing predictive ML models of Random Forest and CatBoost. The BDL-SP model comprehends gene-gene interactions using the PPI network and analyzes genomic features using a deep learning (DL) architecture to identify significantly altered genes and signaling pathways in MM and MGUS. With the help of the BDL-SP model, the researchers could identify the genes and their corresponding enriched signaling pathways that significantly contributed to MM disease development. The BDL-SP model’s findings helped to improve the understanding of cell transformation from pre- malignant to malignant state and strategic diagnosis to support the early detection of transformation to MM. Further investigations are warranted to decipher if any of these differentiating genes could become druggable targets especially during the early phase of MGUS. This has been published in American Journal of Cancer Research 2023;13(4):1155-1187
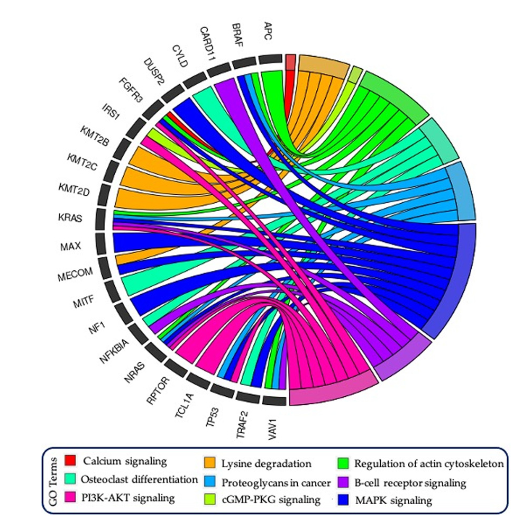
Figure 6: GOChord plot reveals the association of driver/TSG/Onco/Actionable genes associated with important pathways. The gene KMT2C was observed to be significantly mutated in MGUS and MM, while the gene APC was mutated only in MGUS. All other genes were observed to be significantly mutated in MM only.
A collaborative project under Virtual National Cancer Institute (VNCI) initiative led by Tata Memorial Hospital, Mumbai on “Multi-Omics analysis to decipher mechanism of hormone Resistance in Breast Cancer” have standardized a process to establish Patient Derived Xenograft Models from minimum amount of tissue from cancer patient core biopsy. These PDX models will serve as novel in-vivo pre-clinical models for development of novel therapeutics and will constitute a very valuable resource for the researchers and pharmaceutical industry across the country.
Figure 7. Network Program on Hormone Resistant Breast Cancer
A promising NGS based Liquid Biopsy platform has been established where circulating tumour DNA from plasma of cancer patients progressed on endocrine hormone therapy, is subjected to next generation sequencing using a custom amplicon panel for 348 genes. This liquid biopsy platform will enable identification of point mutations in 348 full length genes with high sensitivity and specificity and will be one of the first assays to be developed indigenously to be validated on Indian patients.
Unraveling Molecular Mechanisms for Deeper Cancer Insights
Homologous recombination (HR) plays an essential role in the repair of DNA double-strand breaks (DSBs), replication stress responses, and genome maintenance. However, unregulated HR during replication can impair genome duplication and compromise genome stability. The mechanisms underlying HR regulation during DNA replication are obscure. In one of the study supported at IISc Bangalore, the researchers find that RTEL1 helicase, RAD51, and RAD51 paralogs are enriched at stalled replication sites. The absence of RTEL1 leads to an increase in the RAD51-mediated HRand fork reversal during replication and affects genome-wide replication, which can be rescued by co-depleting RAD51 and RAD51 paralogs. Interestingly, co-depletion of fork remodelers such as SMARCAL1/ZRANB3/ HLTF/FBH1andexpression of HR-defective RAD51 mutants also rescues replication defects in RTEL1-deficient cells. The anti-recombinase function of RTEL1 during replication depends on its interaction with PCNA and helicase activity. Together, this data identified the role of RTEL1 helicase in restricting RAD51-mediated fork reversal and HR activity to facilitate error-free genome duplication. This has been published in Cell Reports, August 2024 issue. (https://doi.org/10.1016/j.celrep.2024.114594)
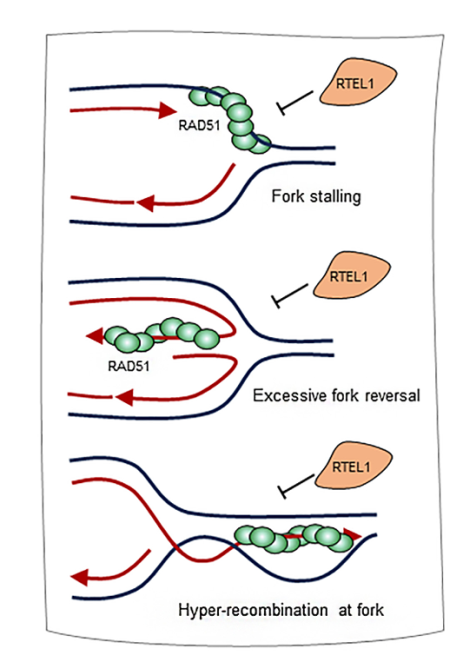
Figure 8 : A model to explain RTEL1functions at the replicating sites RTEL1 regulates HR and fork reversal at stalled replication forks by limiting RAD51 accumulation. This activity of RTEL1 prevents excessive fork remodeling by RAD51 and RAD51-mediated hyper-HR
International Collaboration - India-U.S. Cancer Moonshot Initiative:
During the Official State visit of Prime Minister Shri Narendra Modi to the United States in June 2023, the India-USA Joint Statement reflected that “the leaders committed to holding a U.S.-India Cancer Dialogue, hosted by President Biden’s Cancer Moonshot, to bring experts together from both countries to identify concrete areas of collaboration to accelerate the rate of progress against cancer.”
As a follow up on the joint statement, Department of Biotechnology prepared an initial Concept note on India US Cancer Moonshot Initiative and constituted a high level expert committee called ‘National Cancer Innovation Leadership Initiatives [NCiLi]’. The first meeting of this Committee was held on 17th December 2023 to outline the priorities for collaboration with US agencies and how to strengthen cancer care including underserved urban and rural communities.
The Department of Biotechnology, being a nodal agency, organized several consultation meetings with stakeholder agencies in India to prepare a roadmap to build on the existing resources of an equitable and affordable cancer innovation ecosystem in India and identify the priority areas for the proposed India-US Cancer Moonshot Dialogue.
A one day Lead-up event was held on 28th May, at AIIMS – NCI Jhajjar Campus with more tha 100 participants that included NCiLi members, USHSS officials in US Embassy, representation from MEA, Indian Embassy in Washington, Clinicans and researchers from NCI- AIIMS, Jhajjar Campus and AIIMS, New Delhi , Scientists/Scholars from DBT- BRIC institutions, university and scientific institutions engaged in cancer research across the country.
Subsequently, the first U.S.-India Cancer Moonshot Dialogue was hosted in New Delhi by the Department of Biotechnology, Government of India, on August 5-6, 2024. Over two days, dynamic discussions highlighted the importance of strategic partnership in leveraging emerging technologies for direct interventions aimed at improving cancer care and treatment, with a focus on high-burden, high-mortality cancers. The key recommendations & future course of action are being finalized.

First India-U.S. Cancer Moonshot Dialogue (5-6 August, 2024, New Delhi)
Contacts Concerned Officer for more Information
| Programme Head | Dr. Anamika Gambhir, Scientist G |
|---|---|
| anamika[at]dbt[dot]nic[dot]in | |
| Phone No. | 011-24363665 |
| Associate Head (Cancer) | Phone No. | |
|---|---|---|
| Dr. Niloo Srivastava, Scientist F | nsrivastava[dot]dbt[at]nic[dot]in | 011-24369613 |
















