Indo-U.S. Vaccine Action Programme
Overview
The Indo-US Vaccine Action Programme (VAP) is a bilateral programme, under implementation since 1987, with an aim to support: novel vaccine research, human immunology, vaccine related technologies, translational research and other activities of shared scientific interest supported jointly by both sides. This is a unique programme is being implemented by Department of Biotechnology (DBT), and Indian Council of Medical Research (ICMR) Government of India on the Indian side, in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), on the US side. The oversight to the programme is provided by the Joint Working Group (JWG) comprising of the Experts and the policy makers from India and US.
The VAP is recognized internationally and considered as a model bilateral programme in biomedical research. The VAP, since its inception has supported a large number of collaborative research projects, provided training opportunities for Indian students and scientists, held bilateral workshops on vaccines and infectious diseases.
Thrust Areas

Major Programs
The Indo-US Vaccine Action Programme has also initiated several bilateral programmes under its fold, as follows:

Major Achievements under various initiatives of VAP
1. Candidate Vaccine Advisory Committee
Established in 2016 under the aegis of Indo-U.S. Vaccine Action Program (VAP), the Candidate Vaccine Advisory Committee (CVAC) provides recommendations to VAP leadership concerning the technical aspects of research and development (R&D) related to vaccine candidates approaching readiness for clinical trials in India.
In its recent meetings, CVAC has reviewed vaccine candidates of Dengue, Tuberculosis, Chikungunya, RSV, Influenza and COVID-19 for further support towards their advancement in the development pipeline.
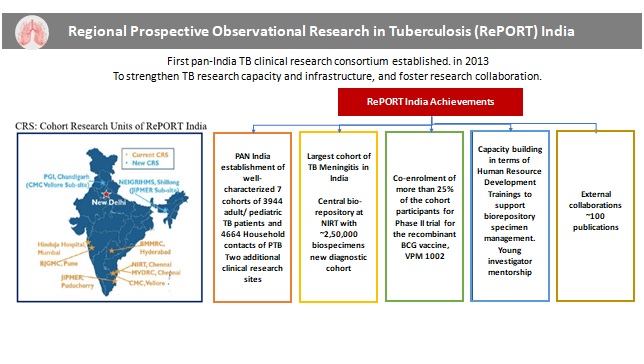
2. RePORT-India (Regional Prospective Observational Research on Tuberculosis)
The Regional Prospective Observational Research in Tuberculosis (RePORT) India initiative, a bilateral collaborative effort is being supported under the aegis of the Indo-US Vaccine Action Programme (VAP), to advance tuberculosis (TB) research in India. The initiative has been under implementation since 2013 with a focus on the establishment of a TB consortium with PAN-India representation, involving long term longitudinal cohorts of TB patients and their household contacts in India.
Basic and applied research for development of newer diagnostics and identification of novel improved biomarkers for targeted therapy are being conducted under the RePORT India initiative. In alignment with the WHO’s framework for global TB control, RePORT India is focusing on the 3rd pillar of ‘Intensified research and innovation’. RePORT India is the first multidisciplinary, multi-institutional clinical and translational TB research consortium for India, whereby nine clinical research sites are being supported. The RePORT sites majorly enrol two sets of cohorts – Patients with active tuberculosis (Cohort A) and their healthy household contacts (Cohort B). A central biorepository is being supported at NIRT, Chennai NIRT.
The initiative has supported co-enrolment of more than 25% of the cohort participants for Phase II trial for the recombinant BCG vaccine, VPM 1002. RePORT India is also supporting capacity building in terms of Human Resource Development by providing trainings to support biorepository specimen management and young investigator mentorship. As an outcome of the projects supported under the RePORT initiative, ~100 publications have been made.
3. Indo-US human immunology programme jointly with Human Immunology Project Consortium (HIPC)
The goal of this funding program is to promote collaborative research between Indian and U.S.-based HIPC investigators to conduct human phenotyping in the context of infectious disease or vaccines of public health importance to both the U.S. and India. The programme is designed to support research efforts as depicted below:

DBT and NIAID sponsored two funding opportunities under this collaboration, first in 2013 and the second in 2018. Five awards were made in each cycle.
Collaborative research on immunology of infectious diseases like Typhoid, Dengue, JEV, HBV and TB have been supported. The projects have resulted in 16 good quality publications.
4. Adjuvant Collaboration
In August 2019, DBT issued a joint funding opportunity announcement with NIAID for collaborative adjuvant research projects to bring together researchers in India and the US. Four applications were selected for funding whereby DBT supports the Indian project and NIAID supports the US project. The pathogens for which vaccines with novel adjuvants are being developed are Mtb, RSV, Flaviviruses, and Foot-and-Mouth Disease.
Success stories under the adjuvant collaboration include the development of Covaxin, the indigenous inactivated COVID-19 vaccine candidate currently being developed by Bharat Biotech, with the adjuvant Alhydroxiquim-II (from US-based ViroVax) that was developed through NIAID’s adjuvant program. The collaboration between Bharat Biotech and ViroVax was initiated during a visit to the biotech companies organized by NIAID together with the adjuvant consultation in 2019.
New Initiative
5. Bioethics Collaboration
Noting the importance of bioethics in the successful conduct of clinical research, the Indo-US VAP Joint Working Group recommended the implementation of a new Initiative to strengthen capacities in biomedical research ethics under the auspices of Indo-US VAP. DBT along with the Department of Bioethics of the NIH Clinical Center is supporting the strengthening clinical research ethics competencies in India. Under this collaboration, the National Biopharma Mission had organized a series of 4 webinars on Clinical Research Ethics. The webinars were attended by more than 1900 attendees. The implementation of a Fellowship programme is under consideration.
Major Success Story
- The Department has supported the development of ROTAVAC®, first indigenously developed rotaviral diarrhoea vaccine, manufactured by M/s Bharat Biotech International Limited, Hyderabad. The vaccine was launched by the Honourable Prime Minister, Shri Narendra Modi in March, 2015. Based on the recommendation of the National Technical Advisory Group on Immunization (NTAGI), the Ministry of Health and Family Welfare has introduced the rotavirus vaccine in the national immunization programme of the country.
- The Department has supported International Centre for Genetic Engineering and Biotechnology (ICGEB), for the development of safe, efficacious and inexpensive tetravalent dengue vaccine (DSV4). The encouraging outcomes in the pre-clinical evaluation of DSV4 enabled its transfer to Sun Pharma, to advance DSV4 to the next stage of development. The candidate is undergoing preclinical toxicity study. They have received funding approval for Phase I Clinical Trial from NBM.Also, Indian Immunologicals Ltd is developing the live attenuated tetravalent dengue vaccine candidate licensed from NIH. The vaccine is being supported for Pre-clinical and Phase I CT material generation by NBM.
- International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi along with its translational research partner “Multi Vaccines Development Program” earlier known as “Malaria Vaccine Development Program” (a not-for-profit society formed and registered under the Societies Registration Act) has advanced the development of blood-stage vaccines for P. falciparum and P. vivax malaria over the last decade. The JAIVAC-2 malaria vaccine project has received funding from the Department of Biotechnology (DBT), Government of India for Manufacturing and Toxicity studies. The Phase I trial finding is committed by BIRAC. The vaccine is undergoing toxicity studies at JRF, VAPI.
Indo-US VAP Last 5 years patents and publications:
2021:
- Acute Phase Proteins Are Baseline Predictors of Tuberculosis Treatment Failure, Kumar NP, Moideen K, Nancy A, Viswanathan V, Thiruvengadam K, Sivakumar S, Hissar S, Kornfeld H, Babu S.. Front Immunol. 2021 Nov 15;12:731878. doi: 10.3389/fimmu.2021.731878. PMID: 34867953; PMCID: PMC8634481
- Effect of anti-tuberculosis treatment on the systemic levels of tissue inhibitors of metallo-proteinases in tuberculosis - Diabetes co-morbidity, Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Hissar S, Kornfeld H, Babu S.. J Clin Tuberc Other Mycobact Dis. 2021 Apr 22;23:100237. doi: 10.1016/j.jctube.2021.100237. PMID: 33997311; PMCID: PMC8100611.
- Targeted next generation WordCleaner directly from sputum for comprehensive genetic information on drug resistant Mycobacterium tuberculosis. Kambli P, Ajbani K, Kazi M, Sadani M, Naik S, Shetty A, Tornheim JA, Singh H, Rodrigues C. Tuberculosis (Edinb). 2021 Mar;127:102051. doi: 10.1016/j.tube.2021.102051. Epub 2021 Jan 8. PMID: 33450448.
- MTB/RIF Ultra over Xpert® MTB/RIF in the diagnosis of extrapulmonary TB” D. J. Christopher, V. Coelho, G. S. Ebby, D. Shankar, R. Gupta, B. Thangakunam, Departments of Pulmonary Medicine, General Surgery - Unit IV, Gastroenterology, and Respiratory Medicine, Christian Medical College, Vellore, India INT J TUBERC LUNG DIS 25(11):939–944 Q 2021. The Union. Nov 2021. DOI: http://dx.doi.org/10.5588/ijtld.21.0280
- Deep LF-Net: Semantic lung segmentation from Indian chest radiographs including severely unhealthy images, Anushikha Singh a, Brejesh Lall b, B.K. Panigrahi b, Anjali Agrawal c, Anurag Agrawal d, Balamugesh Thangakunam , D.J. Christopher, Biomedical Signal Processing and Control, Apr 2021 DOI: https://doi.org/10.1016/j.bspc.2021.102666
- Accuracy of Timika trial scoring system to predict the treatment outcomes among tuberculosis patients in India. Yuvaraj Krishnamoorthy, Selby Knudsen, Sathish Rajaa, Subitha Lakshminarayanan, P.B. Senbagavalli, Jerrold Ellner, Charles Horsburgh, Natasha Hochberg, Padmini Salgame, Sonali Sarkar. IJTB (in press) Received 18 September 2020, Accepted 4 August 2021, Available online 11 August 2021. https://doi.org/10.1016/j.ijtb.2021.08.004.
- Tuberculosis-Learning the trial of Nutrition (TB LION): protocol for an interventional study to decrease TB risk in household contacts. Cintron C, Narasimhan PB, Locks L, Babu S, Sinha P, Rajkumari N, Kaipilyawar V, Bhargava A, Maloomian K, Chandrasekaran P, Verma S, Joseph N, Johnson WE, Wanke C, Horsburgh CR Jr, Ellner JJ, Sarkar S, Salgame P, Lakshminarayanan S, Hochberg NS. BMC Infect Dis. 2021 Oct 12;21(1):1058. doi: 10.1186/s12879-021-06734-z.
- Comparison of profile and treatment outcomes between elderly and non-elderly tuberculosis patients in Puducherry and Tamil Nadu, South India. Murali S, Krishnamoorthy Y, Knudsen S, Roy G, Ellner J, Horsburgh CR, Hochberg N, Salgame P, Prakash Babu S, Sarkar S. PLoS One. 2021 Aug 27;16(8): e0256773.
- Prevalence and factors associated with diabetes mellitus among tuberculosis patients in South India-a cross-sectional analytical study. Sathish Rajaa, Yuvaraj Krishnamoorthy, Selby Knudsen, Gautam Roy, Jerrold Ellner, Charles Horsburgh, Natasha Hochberg, Padmini Salgame, Govindarajan S, Senbagavalli Prakash Babu, Sonali Sarkar. BMJ Open 2021;11:e050542. doi: 10.1136/bmjopen-2021-050542
- 'People listen more to what actors say': A qualitative study of tuberculosis-related knowledge, behaviours, stigma, and potential interventions in Puducherry, India. Sabin LL, Thulasingam M, Carwile M, Babu SP, Knudsen S, Dong L, Stephens J, Fernandes P, Cintron C, Horsburgh CR, Salgame P, Ellner JJ, Sarkar S, Hochberg NS. Glob Public Health. 2021 Oct 16:1-13.
- Prevalence and Risk Factors associated with Latent Tuberculosis Infection among Household Contacts of Smear Positive Pulmonary Tuberculosis patients in South India. Krishnamoorthy Y, Ezhumalai K, Murali S, Rajaa S, Jose M, Sathishkumar A, Govindarajan S, Horsburgh C, Hochberg N, Johnson E, Knudsen S. Tropical Medicine & International Health. 2021 Oct 15. https://doi.org/10.1111/tmi.13693
- Reasons for refusal among patients with tuberculosis and their household contacts to participate in an observational cohort study. Raghupathy Kalaivani, Knudsen Selby, Ellner Jerrold, Horsburg Charles, Hochberg Natasha, Salgame Padmini, Chinnakali Palanivel, Prakashbabu Senbagav alli, Sarkar Sonali. Perspect Clin Res. 2021 Oct-Dec;12(4):234-235. doi: 10.4103/picr. Epub 2021 Sep 20. PMID: 34760653; PMCID: PMC8525795.
- Severe undernutrition in children affects tuberculin skin test performance in Southern India Reddy D, Ma Y, Lakshminarayanan S, Sahu S, White LF, Reshma A, Roy G, Salgame P, Knudsen S, Cintron C, Ellner JJ, Horsburgh CR Jr, Sarkar S, Hochberg NS. Severe undernutrition in children affects tuberculin skin test performance in Southern India. PLoS One. 2021 Jul 16;16(7): e0250304 doi: 10.1371/journal.pone.0250304. PMID: 34270546; PMCID: PMC8284816.
- Comparing Tuberculosis Gene Signatures in Malnourished Individuals using the TB Signature Profiler Johnson WE, Odom A, Cintron C, Muthaiah M, Knudsen S, Joseph N, Jenkins DF, Babu S, Lakshminarayanan S,Zhao Y, Nankya E, Horsburgh CR Jr,Roy G, Ellner JJ, Sarkar S,Salgame P*, Hochberg NS.. BMC Inf Dis 2021; 21(1):106. PMID: 33482742.
- Health-related quality of life and its effect on TB treatment outcomes. Roy N, Krishnamoorthy Y, Rajaa S, Ezhumalai K, Madhusudhanan S, Raghupathy K, Knudsen S, Horsburgh CR, Hochberg NS, Salgame P, Ellner J, Subitha L, Babu SP, Sarkar S. The International Journal of Tuberculosis and Lung Disease, Volume 25 (4) 1 April 2021, pp. 318-320(3) PMID: 33762076. DOI: 10.5588 /ijtld.20.0722
- Reduced thyroxine production in young household contacts of tuberculosis patients increases active tuberculosis disease risk Devalraju KP, Tripathi D, Neela VSK, Paidipally P, Bogam AK, Mallidi V, Sykam A, Singh KP, Ansari MS, Vankayalapati R, Valluri VL. JCI Insight. 2021; 6(13) : e 148271.
- Higher interleukin-6 levels and changes in transforming growth factor-β are associated with lung impairment in pulmonary tuberculosis. Akshay N. Gupte, Sriram Selvaraju, Sanjay Gaikwad, Vidya Mave, Pavan Kumar, Subash Babu, Bruno B. Andrade, William Checkley, Robert Bollinger, and Amita Gupta for the CTRIUMPH trial team ERJ Open Research (European Resipratory Society), 07 January 2021
- Measuring Tuberculosis Drugs in Hair in Adults and Children as a Tool to Monitor Exposure and Outcomes Vidya Mave, Dileep Kadam, Sanjay Gaikwad, Aarti Kinikar, Amol Chavan, Mandar Paradkar, Amol Chavan, Shri Vijay Bala Yogendra, Anju Kagal, Nishi Suryavanshi, Vandana Kulkarni, Kelly E. Dooley, Amita Gupta, Peter Bacchetti, Roy Gerona, Nikhil Gupte, Monica Gandhi. IJTLD, January 2021
- Unhealthy alcohol use is independently associated with unfavorable TB treatment outcomes among Indian men. Cox SR, Gupte AN, Thomas B, Gaikwad S, Mave V, Padmapriyadarsini C, Sahasrabudhe TR, Kadam D, Gupte N, Hanna LE, Kagal A, Paradkar M, Thiruvengadam K, Jain D, Atre S, Sekar K, Raskar S, Shivakumar SVBY, Santhappan R, Deshmukh S, Pradhan N, Kulkarni V, Kakrani A, Barthwal MS, Sawant T, DeLuca A, Suryavanshi N, Chander G, Bollinger R, Golub JE, Gupta A. doi: 10.5588/ijtld.20.0778. PMID: 33688806. IJTLD, March 2021
- Host Lipidome Predicts Tuberculosis Treatment Failure Rupak Shivakoti, John W Newman, Luke Elizabeth Hanna, Artur T L Queiroz, Kamil Borkowski, Akshay N Gupte, Mandar Paradkar, Pattabiraman Satyamurthi, Vandana Kulkarni, Murugesh Selva, Neeta Pradhan, Shri Vijay Bala Yogendra Shivakumar, Saravanan Natarajan, Ramesh Karunaianatham, Nikhil Gupte, Kannan Thiruvengadam, Oliver Fiehn, Renu Bharadwaj, Anju Kagal, Sanjay Gaikwad, Shashikala Sangle, Jonathan E. Golub, Bruno B Andrade, Vidya Mave, Amita Gupta, Padmapriyadarshini Chandrasekaran. DOI: 10.1183/13993003.04532-2020 European Respiratory Journal; May 2021
- Baseline IL-6 is a biomarker for unfavorable tuberculosis treatment outcomes: a multi-site discovery and validation study.|
- Akshay N. Gupte, Pavan Kumar, Mariana Araújo-Pereira, Vandana Kulkarni, Mandar Paradkar, Neeta Pradhan, Padmapriya Darasini, Luke Hanna, Shri Vijay Bala Yogendra Shivakumar, Neesha Rockwood, Elsa Du Bruyn, Rajesh Karyakarte, Sanjay Gaikwad, Robert Bollinger, Jonathan Golub, Nikhil Gupte, Vijay Viswanathan, Robert J. Wilkinson, Vidya Mave, Subash Babu, Hardy Kornfeld, Bruno B. Andrade, Amita Gupta. European Respiratory Journal October 2021
- Potential Drug-drug Interactions between Anti–tubercular and Non-tubercular drugs among Patients with Pulmonary Tuberculosis in Puducherry and Tamil Nadu. Cathrine John Marie, Senbagavalli PB, Komala Ezhumalai, Selby Knudsen, C. Robert Horsburgh, Natasha S. Hochberg, Padmini Salgame, Jerrold Ellner, Sonali Sarkar. European Journal of Pharmaceutical and Medical Research (EJPMR). 2021,8(1), 427-440.
- Food for thought: addressing undernutrition to end TB. Sinha P, Lönnroth K, Bhargava A, Heysell SK, Sarkar S, Salgame P, Rudgard W, Boccia D, Van Aartsen D, Hochberg NS. Lancet InfectDis. 2021 Oct;21(10):e318-e325. doi: 10.1016/S1473-3099(20)30792-1. Epub 2021 Mar 23. PMID: 33770535; PMCID: PMC8458477.
- Concomitant Pulmonary Disease is Common among Indian Patients With Extrapulmonary Tuberculosis. Shri Vijay Bala Yogendra, Shivakumar, Chandrasekaran Padmapriyadarshini, Amol Chavan, Mandar Paradkar, BM Shrinivasa, Akshay Gupte, Kavitha Dhanasekaran, Beena Thomas, Nishi Suryavanshi, Chandra Kumar Dolla, Sriram Selvaraju, Aarti Kinikar, Sanjay Gaikwad, Rewa Kholi, Gomathi Narayan Sivaramakrishnan, Neeta Pradhan, Luke Elizabeth Hanna, Vandana Kulkarni, Andrea DeLuca, Samyra R. Cox, Lakshmi Murali, Kannan Thiruvengadam, Swapnil Raskar, Geeta Ramachandran, Jonathan E. Golub, Nikhil Gupte, Vidya Mave, Soumya Swaminathan MD, Amita Gupta, Robert C Bollinger for the CTRIUMPh RePORT India Study Team. IJTLD, November 2021 (Accepted and In-Press)
- Whole Genome Sequencing Assessing Impact of Diabetes Mellitus on Tuberculosis Mutations and Type of Recurrence in India Vidya Mave, Liang Chen, Umadevi Ranganathan, DileepKadam, Rahul Lokhande, Shivkumar, Anju Kagal, Neeta Pradhan, Vijay Vishwanathan, Amita Gupta, Padmapriyadarshini, Jonathan E. Golub, Nikhil Gupte, Barun Mathema, Barry Kreiswirth CID (Reviewer response being addressed, December, 2021)
- Modern WordCleaner of Mycobacterium tuberculosis were recently introduced in western India and demonstrate increased transmissibility, Avika Dixit, Anju Kagal, Yasha Ektefaie, Luca Freschi, Rahul Lokhande, Matthias Groeschel, Jeffrey A. Tornheim, Nikhil Gupte, Neeta Pradhan, Deelip Kadam, Marco Schito, David Engelthaler, Amita Gupta, Jonathan Golub, Vidya Mave, Maha Farhat. In progress (Under co-author review)
- Sex differences in tuberculosis clinical presentation, drug exposure, and treatment outcomes in Pune, India. Sona Deshmukh, Manasi Sane, Sanjay Gaikwad, Tushar Sahasrabudhe, Madhusudan Barthwal, Rahul Lokhande, Swapnil Raskar, Anju Kagal, Sujata Dharmshale, Neeta Pradhan, Akshay Gupte, Omamah Alfarisi, Amita Gupta, Kelly E. Dooley, Nikhil Gupte, Jonathan E. Golub, Vidya Mave. Chest, November 2021 (under Journal review)
- Aggarwal C, Saini K, Reddy ES, Singla M, Nayak K, Chawla YM, Maheshwari D, Singh P,Sharma P, Bhatnagar P, Kumar S, Gottimukkala K, Panda H, Gunisetty S, Davis CW, Kissick HT, Kabra SK, Lodha R, Medigeshi GR, Ahmed R, Murali-Krishna K, Chandele A. Immunophenotyping and transcriptional profiling of human plasmablasts in dengue. J Virol. 2021 Sep 15:JVI0061021. doi: 10.1128/JVI.00610-21.
- Nayak K, Gottimukkala K, Kumar S, Reddy ES, Edara VV, Kauffman R, Floyd K, Mantus G, Savargaonkar D, Goel PK, Arora S, Rahi M, Davis CW, Linderman S, Wrammert J, Suthar MS, Ahmed R, Sharma A, Kaja MK, Chandele A. Characterization of neutralizing verus binding antibodies and memory B cells in COVID-19 recovered individuals. Virology, 2021, 13-21.
2020:
- Clinical features associated with linezolid resistance among multidrug resistant tuberculosis patients at a tertiary care hospital in Mumbai, India, Tornheim JA, Intini E, Gupta A, Udwadia ZF. Journal of clinical tuberculosis and other mycobacterial diseases vol. 20 100175. 24 Jul. 2020, doi:10.1016/j.jctube.2020.100175
- WordCleaner genome enrichment approach for rapid detection of Mycobacterium tuberculosis and drug resistance-associated mutations from direct sputum sequencing, Soundararajan L , Kambli P , Priyadarshini S, Let B, Murugan S, Iravatham C,Tornheim JA ,Rodrigues C, Gupta R, Ramprasad VL. Tuberculosis Volume 121, March 2020, 101915. https://doi.org/10.1016/j.tube.2020.101915.
- Association of Plasma Matrix Metalloproteinase and Tissue Inhibitors of Matrix Metalloproteinase Levels With Adverse Treatment Outcomes Among Patients With Pulmonary Tuberculosis, Kumar, N.P., Moideen, K., Nancy, A., Viswanathan, V., Thiruvengadam, K., Nair, D., Banurekha, V.V., Sivakumar, S., Hissar, S., Kornfeld, H. & Babu, S. (2020).. JAMA Netw Open 3(12): e2027754.
- Impact of diabetes and low body mass index on tuberculosis treatment outcomes, Kornfeld H, Sahukar BS, Procter-Gray E, Kumar NP, West K, Kane K, Natarajan M, Li W, Babu S, Viswanathan V. Clin Infect Dis 2020 Dec 3;71(9):e392-e398. doi: 10.1093/cid/ciaa054.PMID: 31955202.
- Plasma chemokines are baseline predictors of unfavorable treatment outcomes in pulmonary tuberculosis, Nathella P Kumar, Kadar Moideen, Arul Nancy, Vijay Viswanathan, Kannan Thiruvengadam, Dina Nair, Vaithilingam V Banurekha, Shanmugam Sivakumar, Syed Hissar, Hardy Kornfeld, Subash Babu. Clin Infect Dis (epub). doi: 10.1093/cid/ciaa1104. PMID: 32766812; PMCID: PMC8563183.
- Heterogeneity in the cytokine profile of tuberculosis-diabetes co-morbidity, Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, Natarajan M, Kornfeld H, Babu S. Cytokine 2020 Jan;125:154824. PMID: 31472402.
- Household food insecurity among patients with pulmonary tuberculosis and its associated factors in South India: a cross-sectional analysis, Reshma Ayiraveetil ,1 Sonali Sarkar,1 Palanivel Chinnakali,1 Kathiresan Jeyashree,2 Mathavaswami Vijayageetha,1 Pruthu Thekkur,3,4 Subitha Lakshminarayanan,1 Selby Knudsen,5 Natasha S Hochberg,5,6 C Robert Horsburgh,5,6,7 Jerrold Ellner,8 Gautam Roy. 2020. Ayiraveetil R, et al. BMJ Open 2020;10:e033798. doi:10.1136/bmjopen-2019-033798
- “Deep Learning for Automated Screening of Tuberculosis from Indian Chest X-rays: Analysis and Update”, Anushikha Singh, Brejesh Lall, B.K. Panigrahi, Anjali Agrawal, Anurag Agrawal, Balamugesh Thangakunam, DJ Christopher. Journal of Thoracic Imaging, Nov 2020. DOI: https://arxiv.org/abs/2011.09778
- “Xpert Ultra Is Better Than, Xpert, but Using Biopsy, Samples May Be Even Better”. Balamugesh Thangakunam, MD, DM, FCCP, Devasahayam Jesudas Christopher, DNB, FCCP. Vellore, India. Chest. Aug 2020. DOI: https://journal.chestnet.org/article/S0012-3692(20)30760-1/fulltext
- Screening for prevalence of trial TB disease and latent TB infection in type 2 diabetes mellitus patients attending a diabetic clinic in an Indian tertiary care hospital. Pradipkumar Arvindbhai Dabhi, Thangakunam Balamugesh, RichaGupta, Devasahayam Jesudas Christopher. PLoS ONE 15(6):e0233385, June 2020. DOI: 10.1371/journal.pone.0233385.
- Burden of diabetes among patients with tuberculosis: 10 year experience from a tertiary care referral teaching hospital in South India, Christopher DJ, Jeyaseelan L, Yadav B, Balaji V, Michael JS, Gupta M, Manipadam MT, Sudarsanam TD. May 2020. DOI: 10.4103/ lungindia lungindia_111_19
- BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice. Radhakrishnan RK, Thandi RS, Tripathi D, Paidipally P, McAllister MK, Mulik S, Samten B, Vankayalapati R. JCI Insight. 2020 Mar 12;5(5):e133788. doi: 10.1172/jci. insight. 133788. PMID: 32161191; PMCID: PMC7141407.
- Metabolites enhance innate resistance to human Mycobacterium tuberculosis infection. Deepak Tripathi, Kamakshi Prudhula Devalraju, Venkata Sanjeev Kumar Neela, Tanmoy Mukherjee, Padmaja Paidipally, Rajesh Kumar Radhakrishnan, Igor Dozmorov, Mohammad Soheb Ansari, Varalakshmi Mallidi, Anvesh Kumar Bogam, Vijaya Lakshmi Valluri, Ramakrishna Vankayalapati. (JCI Insight – first revision).
- Tuberculosis Preventive Treatment Should be Considered for All Household Contacts of pulmonary TB Patients in India. Mandar Paradkar, Chandrasekaran Padmapriyadarsini, Divyashri Jain, Shri Vijay Bala Yogendra Shivakumar, Kannan Thiruvengadam, Akshay Gupte, Beena Thomas, Aarti Kinikar, Krithika Sekar, Renu Bharadwaj, Chandra Kumar Dolla, Sanjay Gaikwad, S.Elilarasi, Rahul Lokhande, Devarajulu Reddy, Lakshmi Murali, Vandana Kulkarni, Neeta Pradhan, Elizabeth Hanna, Sathyamurthi Pattabiraman, Rewa Kohli, Rani Nayagam, Nishi Suryavanshi, Shrinivasa BM, Samyra Cox, Sriram Selvaraju, Nikhil Gupte, Vidya Mave, Amita Gupta, Robert C. Bollinger, for the CTRIUMPH-RePORT India Study Team, PLOS One, 29 July 2020
- Assessment of persistent depression among Tuberculosis patients in India (Letter), Nishi Suryavanshi, Manasi Sane, Sanjay Gaikwad, Mandar Paradkar, Vidya Mave, Padmapriyadarsini Chandrasekaran, Shri Vijay Bala Yogendra Shivakumar, Amita Gupta, Nikhil Gupte, Beena Thomas for CTRIUMPH RePORT India study (https://doi.org/10.5588/ijtld.20.0231) IJTLD, November 2020
- Identification of metabolomics and transcriptomics reveals novel biomarkers in blood for tuberculosis in children. Noton K. Dutta, Jeffrey A. Tornheim, Kiyoshi F. Fukutani, Mandar Paradkar, Rafael T. Tiburcio, Aarti Kinikar, Chhaya Valvi, Vandana Kulkarni, Neeta Pradhan, Shri Vijay Bala Yogendra Shivakumar, Anju Kagal, Akshay Gupte, Nikhil Gupte, Vidya Mave, Amita Gupta, Bruno B. Andrade, and Petros C. Karakousis for the CTRIUMPh RePORT India Study Team. Scientific Reports (nature), November 2020
- Higher IL-6 trial and Changes in TGF-β are Associated with Lung Impairment in Pulmonary Tuberculosis. AkshayN Gupte, Sriram Selvaraju, Sanjay Gaikwad, Vidya Mave, Pavan Kumar, Subash Babu, Bruno B. Andrade, William Checkley, Robert Bollinger, Amita Gupta for the CTRIUMPH study team, ERJ Open Research 2020; DOI: 10.1183/23120541.00390-2020, ERJ OpenResearch (European Resipratory Society), November 2020
2019
- Delamanid central nervous system pharmacokinetics in tuberculous meningitis in rabbits and humans, Tucker EW, Pieterse L, Zimmerman MD, Udwadia ZF, Peloquin CA, Gler MT, Ganatra S, Tornheim JA, Chawla P, Caoili JC, Ritchie B, Jain SK, Dartois V, Dooley KE. AntimicrobAgents Chemother. 2019 Sep 23;63(10). pii: e00913-19. doi: 10.1128/AAC.00913-19. Print 2019 Oct. PMID: 31383662; PMCID: PMC6761520.
- Management of drug-resistant tuberculosis, Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr. Lancet. 2019 Sep 14;394(10202):953-966. doi: 10.1016/S0140-6736(19)31882-3. PMID: 31526739.
- Multi-centre study to establish interpretive criteria for clofazimine drug susceptibility testing,Ismael N, Said HM, Rodrigues C ,Omar SV, Ajbani K, Sukhadia N, Kohl TA, Niemann S, Kranzer K,Diels M, Rigouts L, Rüsch-Gerdese S, Siddiqi S. Int J Tuberc Lung Dis. 2019 May 1;23(5):594-599. doi: 10.5588/ijtld.18.0417. PMID: 31097068.
- Performance of bioMérieux Lowenstein-Jensen slopes in plastic tube packaging, compared to existing phenotypic methods, for efficient recovery of Mycobacterium tuberculosis (MTB)complex, Nambiar R, Bereksi N, Gonzalez R, Anthony de Cozar, Loubet M, Shetty A, Alex van Belkum, Rodrigues C. J Med Microbiol. 2019 Mar;68(3):398-401. doi: 10.1099/jmm.0.000930. Epub 2019 Feb 6. PMID: 30724723.
- Systemic RAGE trial are upregulated in tuberculosis individuals with diabetes co-morbidity and modulated by anti-tuberculosis treatment and metformin therapy, Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, Hissar S, Kornfeld H, Babu S. BMC Infect Dis 2019 Dec 9;19(1):1039. PMID: 31818258; PMCID: PMC6902343.
- Plasma chemokines are biomarkers of disease severity, higher bacterial burden and delayed sputum culture conversion in pulmonary tuberculosis, Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Sivakumar S, Natarajan M, Kornfeld H, Babu S. Sci Rep 2019 Dec 3;9(1):18217. PMID: 31796883; PMCID: PMC6890773.
- Plasma eicosanoid levels in tuberculosis and tuberculosis-diabetes co-morbidity are associated with lung pathology and bacterial burden, Kumar NP, Moideen K, Nancy A, Viswanathan V, Shruthi BS, Shanmugam S, Hissar S, Kornfeld H, Babu S. Front Cell Infect Microbiol 2019 Oct 1;9:335. PMID: 31632923; PMCID: PMC6779700.
- Persistent inflammation during anti-tuberculosis treatment with diabetes comorbidity, Kumar NP, Fukutani KF, Shruthi BS, Alves T, Silveira-Mattos PS, Rocha MS, West K, Natarajan M, Viswanathan V, Babu S, Andrade BB, Kornfeld H. eLife. 2019 Jul 4;8. pii: e46477. PMID: 31271354; PMCID: PMC6660216.
- Elevated circulating levels of monocyte activation markers among tuberculosis patients with diabetes co-morbidity, Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Natarajan M, Kornfeld H, Babu S. Immunology. 2019 Mar;156(3):249-258. PMID: 30427060; PMCID: PMC6376263.
- Interaction of nutritional status and diabetes on active and latent tuberculosis: a cross-sectional analysis Kubiak R, Sarkar S, Horsburgh CR Jr, Roy G, Kratz M, Reshma, Knudsen S, Salgame P, Ellner JJ, Drain PK, Hochberg NS. BMC Infect Dis. 2019 Jul16;19(1):627. doi: 10.1186/s12879-019-4244-4. PMID: 31311495; PMCID: PMC6636094.
- Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis, Hoyt K, Sarkar S, White LF, Joseph NM, Salgame P, Lakshminarayanan S, Muthaiah M, Kumar SV, Ellner, JJRoy G, Horsburgh CR Jr, Hochberg NS. PLoS ONE. 2019 Mar 27; 14(3). doi: 10.1371/journal.pone.0214011. PMID: 30917170; PMCID: PMC6436704.
- IL-22 produced by type 3 innate lymphoid cells (ILC3s) reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis, Tripathi D, Radhakrishnan RK, Sivangala Thandi R, Paidipally P, Devalraju KP, Neela VSK, McAllister MK, Samten B, Valluri VL, Vankayalapati R. PloS Pathog. 2019 Dec 6;15(12):e1008140. PMID: 31809521
- Respiratory Health Status is Associated with Treatment Outcomes in Pulmonary Tuberculosis. Gupte AN, Selvaraju S, Paradkar M, Danasekaran K, Shivakumar SVBY, ThiruvengadamK, Dolla C, Shivaramakrishnan G, Pradhan N, Kohli R, John S, Raskar S, Jain D, Momin A, Subramanian B, Gaikwad A, Lokhande R, Suryavanshi N, Gupte N, Salvi S, Murali L, Checkley W, Golub JE, Bollinger R, Chandrasekaran P, Mave V, Gupta A. doi: 10.5588/ijtld.18.0551. IJTLD, April 2019
- Assessment of Lung Function in Successfully Treated Tuberculosis Reveals High Burden of Ventilatory Defects and COPD. Gupte A; Paradkar A; Selvaraju S; Thiruvengadam K; Shivakumar SVBY; Sekar K; Marinaik S; Momin A; Gaikwad A; Natrajan P; Prithivi M; Shivaramakrishnan G; Pradhan N; Kohli R; Raskar S; Jain D; Velu R; Karthavarayan B; Lokhande R; Suryavanshi N; Gupte N; Murali L; Salvi S; Checkley W; Golub J; Bollinger R; Mave V; Chandrasekaran P; Gupta A. PLOS One, May 2019
- Sub-therapeutic rifampicin concentration is associated with unfavourable tuberculosis treatment outcomes. Ramachandran G, Chandrasekaran P, Gaikwad S, Kumar AKH, Thiruvengadam K, Gupte N, Paradkar M, Dhanasekaran K, Sivaramakrishnan GN, Kagal A, Thomas B, Pradhan N, Kadam D, Hanna LE, Balasubramanian U, Kulkarni V, Murali L, Golub J, Gupte A, Shivakumar SVBY, Swaminathan S, Dooley KE, Gupta A, Mave V for C-TRIUMPh team. (Clinical Infectious Diseases, ciz380, https://doi.org/10.1093/cid/ciz380), Clinical Infectious Diseases, May 2019
- Age-specific trial of Tuberculosis Infection in Household Contacts in an Endemic setting: Time for Prophylaxis?Dolla CK, Chandrasekaran P,Thiruvengadam K,Lokhande R,Kinikar A, Paradkar M, Gupte A, Gaikwad S, Pradhan N, Kulkarni V, Shivakumar SVBY, Suryavanshi N, Gupte N, Pattabiraman S, Kagal A, Shrinivas B M, Murali L, Bharath TK1, Pirthivi M, Kumaran P, Mave V, Gupta A. TRSTMH, June 2019
- Smoking, Alcohol use disorder and TB treatment outcomes: A dual co-mobidity burden that cannot be ignored. Thomas B , Thiruvengadam K, Rani S, Kadam D, Ovung S, Sivakumar S, Shivakumar SVBY, Paradkar M, Gupte N, Suryavanshi N, Akshay GN, Kohli R, Pradhan N, Sivaramakrishnan GN, Gaikwad S, Kagal A, Dhanasekaran K, Deluca A, Golub JE, Mave V, Chandrasekaran P, Gupta A for the CTRIUMPH- RePORT India Study. PLOS July 2019
- Infection free “Resisters” among Household Contacts of Adult Pulmonary Tuberculosis, Mave V; Chandrasekaran P; Chavan A; Shivakumar SVBY; Danasekaran K; Paradkar M; Thiruvengadam K, Kinikar A; Murali L; Gaikwad S; Hannah LE; Kulkarni V; Pattabiraman S; Suryavanshi N; Thomas B; Kohli R; Sivaramakrishnan GN; Pradhan N; Banu B; Kagal A; Golub J; Gupte A; Gupte N; Swaminathan S; Gupta A. PLOS July 2019
- Lack of association between TIRAP variants and disease severity among the active tuberculosis patients from South India. Rajagopalan S, Pattabiraman S, Thiruvengadam K, Selvachithiram M, Shivakumar SVBY, Sivaramakrishnan GN, Dhanasekaran K, Paradkar M, Puvaneshwari R, Muthuramalingam K, Madheswaran A, Pradhan N, Kulkarni V, Gupte AN , Gupte N, Mave V, Gupta A, Chandrasekaran P, Hanna LE for the C-TRIUMPh Study Team. Infection, Genetics and Evolution, October 2019
- Transcriptomic Profiles of Confirmed Pediatric Tuberculosis Patients and Exposed Household Contacts Identifies Tuberculosis disease, infection, and Response to Treatment Among Indian Patients. Tornheim JA, Madugundu A, Paradkar M, Gupte N, Fukutani KF, Gupte AN, Kinikar A, Kulkarni V, Balasubramanian U, Sreenivasamurthy S, Raja R, Pradhan N, Shivakumar SVBY, Valvi C, Hanna LE, Andrade B, Chandrasekaran P, Mave V, Pandey A, Gupta A for the CTRIUMPh RePORT India Study Team. Journal of Infectious Diseases, December 2019
- Rakshit S+; Ahmed A+; Adiga V+; Sounderaj B+; Sampatkumar R+; Finak G*; Gottardo R*; Stuart K*; De Rosa S*; McElrath J** and Vyakarnam A+**. BCG revaccination of young adults in India induces / expands circulating frequencies of Mtb-specific innate and memory T-cells that likely differ from those induced by infection **Equal Joint Senior Authors
+ Indian Institute of Science, Bangalore, India; * Fred Hutchinson Cancer Research Centre, Seattle, USA
2018
- Capacity for advances in tuberculosis research; proceedings of the third Building RePORT international meeting, Yuri F van der Heijden, Fareed Abdullah, Bruno Bezerril Andrade, Dev asahayam J Christopher [...]Carol Hamilton. Tuberculosis, Oct 2018 DOI: 10.1016/j.tube 2018.09.009.
- Utility of pyrosequencing for rapid detection of tubercular meningitis (TBM) and associated susceptibility directly from CSF specimens, Ajbani K, Kazi M, Naik S, Soman R, Shetty A, Rodrigues C. Tuberculosis (Edinb). 2018 Jul;111:54-56. doi: 10.1016/j.tube.2018.05.009. Epub 2018 May 19. PMID: 30029915
- Evaluation of pyrosequencing for extensive drug resistance-defining anti-tuberculosis drugs for use in public healthcare, Nambiar R, Shah D, Ajbani K, Kazi M, Sadani M, Shetty A, Keskar P, Kamble S, van Belkum A, Rodrigues C. Tuberculosis (Edinb). 2018 May;110:86-90. doi: 10.1016/ j.tube.2018.03.006. Epub 2018 Mar 26. PMID: 29779779
- Pyrosequencing to resolve discrepant Xpert MTB/RIF and Mycobacterial Growth Indicator Tube 960, Ajbani K, Kazi M, Tornheim J, Naik S, Soman R, Shetty A, Rodrigues C. Lung India. 2018 Mar-Apr;35(2):168-170. doi: 10.4103/lungindia.lungindia_71_17. PMID: 29487256; PMCID: PMC5846270.
- Elevated levels of matrix metalloproteinases reflect severity and extent of disease in tuberculosis-diabetes co-morbidity and are predominantly reversed following standard anti-tuberculosis or metformin treatment, Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Menon PA, Kornfeld H, Babu S. BMC Infect Dis. 2018 Jul 25;18(1):345. PMID: 30045688; PMCID: PMC6060542
- Thoracoscopic trial biopsy improves yield of Xpert MTB/RIF for diagnosis of pleural tuberculosis, Christopher DJ, Dinakaran S, Gupta R, James P, Isaac B, Thangakunam B. Respirology . 2018 Jul;23(7):714-717. Epub 2018 Feb 27. PMID: 29486527 DOI: 10.1111/ resp. 13275.
- Existing blood transcriptional classifiers accurately discriminate active tuberculosis from latent infection in individuals from South India, Leong, S, Yue Zhao, Joseph NM, Hochberg NS, Sarkar S, Pleskunas J, HomD, LakshminarayananS, Horsburgh Jr, CR, Roy G, Ellner JJ, Johnson WE, Salgame, P. Tuberculosis (Edinb). 2018 Mar;109:41-51. doi: 10.1016/ j.tube. 2018.01.002. Epub 2018 Jan 31. PMID: 29559120.
- Alcohol WordCleaner type 1 interferon-α production and mortality of young mice infected with Mycobacterium tuberculosis, Tripathi D, Welch E, Cheekatla SS, Radhakrishnan R, Venkatasubramanian S, Paidipally P, Van A, Samten B, Devalraju P, Neela V, Valluri V, Mason C, Nelson S and Vankayalapati R. PloS Pathog. 2018 Aug 2;14(8):e1007174. Doi: 10.1371/ journal.ppat.1007174. eCollection 2018 Aug. PMID: 30071107; PMCID: PMC6072099.
- IL-17 and IL-22 production in HIV+ individuals with latent and active tuberculosis Devalraju KP, Neela VSK, Ramaseri SS, Van A, Chaudhury A, Krovvidi SS, Vankayalapati R, Valluri VL. BMC Infect Dis. 2018 Jul 11;18(1):321. doi: 10.1186/s12879-018-3236-0. PMID: 29996789; PMCID: PMC6042451.
- Defective MyD88 and TRIAL but not TLR-2 expression in HIV+ individuals with latent tuberculosis infection Devalraju KP, Neela VSK, Gaddam R, Chaudhury A, Van A, Krovvidi SS, Vankaylapati R, Valluri VL. Cytokine. 2018 Oct;110:213-221. doi: 10.1016/j.cyto.2018.05.005. Epub 2018 May 17. PMID: 29778672; PMCID: PMC6103807.
- Interleukin-21 regulates trial Killer cell responses during mycobacterium tuberculosis infection, Paidipally P, Tripathi D, Van A, Radhakrishnan RK, Dhiman R, Venkatasubramanian S, Devalraju KP, Tvinnereim AR, Valluri VL, Vankayalapati R. J Infect Dis. 2018 Mar 28;217(8):1323-1333. doi: 10.1093/infdis/jiy034. PMID: 29390153; PMCID: PMC6018723.
- Addressing Knowledge gaps and prevention for tuberculosis-infected Indian adults: a vital part of elimination, DeLuca A, Dhumal G, Paradkar M, Suryavanshi N, Mave V, Kohli R, Shivakumar SVBY, Hulyolkar V, Gaikwad A, Nangude A, Pardeshi G, Kadam D, Gupta A, BMC Infectious disease, May 2018
- Diabetes and Prediabetes among Household Contacts of TB Patients in India: Is it time to screen them all? Shivakumar SVBY, Chandrasekaran P, Kumar AMV, Paradkar M, Dhanasekaran K, Suryavarshini N, Thomas B, Kohli R, Thiruvengadam K, Kulkarni V, Hannah LE, Sivaramakrishnan GN, Pradhan N, Dolla C, Gupte A, Ramachandran G, DeLuca A, Meshram S, Bhardawaj R, Bollinger RC, Golub J, Selvaraj K, Gupte N, Swaminathan S, Mave V, Gupta A. Int J Tuberc Lung Dis. 2018 Jun 1;22(6):686-694. doi: 10.5588/ijtld.17.0598. IJTLD, June 2018
- Tuberculin Skin Test and QuantiFERON-Gold In tube assay for Diagnosis of Latent TB Infection among Household Contacts of Pulmonary TB patients in a high TB burden setting. Chandrasekaran P, Mave V, Thiruvengadam K, Gupte N, Shivakumar SVBY, Hanna LE, Kulkarni V, Kadam D, Dhanasekaran K, Paradkar M, Thomas B, Kohli R, Dolla C, Bharadwaj R, Sivaramakrishnan GN, Pradhan N, Gupte A, Murali L, Valvi C, Swaminathan S, Gupta A; CTRIUMPH Study Team. doi: 10.1371/journal.pone.0199360. PLOS One, August 2018
- Rathore DK, Holmes TH, Nadeau KC, Mittal P, Batra A, Rosenberg-Hasson Y, Sopory S, Gupta R, Chellani HK, Aggarwal KC, Bal V, Natchu UCM, Bhatnagar S, Tavassoli M, Lyell DJ, Rath S, Wadhwa N#, Maecker HT#. Differences in multiple immune parameters between Indian and U.S. infants.PLoS One.2018 Nov 16;13(11):e0207297. doi: 10.1371/journal.pone.0207297. eCollection 2018.
2017
- Heightened circulating levels of antimicrobial peptides in tuberculosis-diabetes co-morbidity and reversal upon treatment, Kumar NP, Moideen K, Viswanathan V, Sivakumar S, Menon PA, Kornfeld H, Babu S. PLoS One. 2017 Sep 14;12(9):e0184753. doi: 10.1371/journal.pone. 0184753. eCollection 2017. PMID: 28910369; PMCID: PMC5599016.
- Systems immunology of diabetes tuberculosis comorbidity reveals signatures of disease complications, Prada-Medina CA, Fukutani KF, Kumar NP, GilSantana L, Babu S, Lichtenstein F, West K, Sivakumar S, Menon PA, Viswanathan V, Andrade BB, Nakaya HI, Kornfeld H. Sci Rep. 2017 May 17;7(1):1999. PMID: 28515464; PMCID: PMC5435727.
- Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 2: Underlying biological mechanisms, Ronacher K, van Crevel R, Critchley J, Bremer A, Schlesinger LS, Kapur A, Basaraba R, Kornfeld H, Restrepo BI. Chest. 2017 Jul;152(1):174-180. PMID: 28434937; PMCID: PMC5577357.
- Defining a research agenda to address the converging epidemics of tuberculosis and diabetes. Part 1: Epidemiology and clinical management, Critchley JA, Restrepo BI, Ronacher K, Kapur A, Bremer AA, Schlesinger LS, Basaraba R, Kornfeld H, van Crevel R. Chest. 2017 Jul;152(1):165-173. PMID: 28434936; PMCID: PMC5989639.
- Tuberculosis-diabetes co-morbidity is characterized by heightened systemic levels of circulating angiogenic factors, Kumar NP, Moideen K, SivakumarS, Menon PA, Viswanathan V, Kornfeld H, Babu S. J Infect. 2017 Jan;74(1):10-21. PMID: 27717783; PMCID: PMC5164955.
- Comorbidities in pulmonary tuberculosis cases in Puducherry and Tamil Nadu, India: Opportunities for intervention, Hochberg NS, Sarkar S, Horsburgh, Jr, CR, Knudsen, Pleskunas J, Sahu S, Kubiak RW, Govindarajan S, Salgame P, Lakshminarayanan S, Sivaprakasam A, White LF, Joseph NM, Ellner JJ, Roy G. PLoS One. 2017 Aug 23;12(8):e0183195. doi: 10.1371/ journal.pone.0183195. eCollection 2017. PMID: 28832615; PMCID: PMC5568341.
- Predictors of delayed care seeking for tuberculosis in Southern India: An observational study, Van Ness SE, Chandra A, Sarkar S, Pleskunas J, Ellner JJ, Roy G, Lakshminarayanan S, Sahu S, Horsburgh Jr CR, Jenkins HE, Hochberg NS. BMC Infect Dis. 2017 Aug 15;17(1):567. doi: 10.1186/s12879-017-2629-9. PMID: 28806911; PMCID: PMC5557420.
- Defective expansion and function of memory like natural killer cells in HIV+ individuals with latent tuberculosis infection. Kamakshi Prudhula Devalraju, Venkata Sanjeev Kumar Neela, Siva Sai Krovvidi, Ramakrishna Vankayalapati, Vijaya Lakshmi Valluri PLoS ONE 16(9): e0257185.
- IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis, Venkatasubramanian S, Cheekatla S, Paidipally P, Tripathi D, Welch E, Tvinnereim AR, Nurieva R, Vankayalapati R. Mucosal Immunol. 2017 Jul;10(4):1031-1042. doi: 10.1038/mi.2016.105. Epub 2016 Dec 7. PMID: 27924822; PMCID: PMC5462891.
- JaNin J, ayak K, Tanwar N, Gaind R, Gupta B, Shastri JS, Bhatnagar RK, Kaja MK, Chandele A, Sunil S. Clinical, Serological, and Virological Analysis of 572 Chikungunya Patients From 2010 to 2013 in India. Clin Infect Dis. 2017 Jul 1;65(1):133-140. doi: 10.1093/cid/cix283. PubMed PMID: 28379375.
2016
- NK-CD11c+ cell crosstalk in diabetes enhances IL-6-mediated inflammation during Mycobacterium tuberculosis infection, Cheekatla SS, Tripathi D, Venkatasubramanian S, Nathella PK, Paidipally P, Ishibashi M, Welch E, Tvinnereim AR, Ikebe M, Valluri VL, Babu S, Kornfeld H, Vankayalapati R. PLoS Pathog. 2016 Oct 26;12(10):e1005972. doi: 10.1371/journal. ppat.1005972. eCollection 2016. PMID: 27783671; PMCID: PMC5082658.
- Cohort for Tuberculosis Research by the Indo-US Medical Partnership (CTRIUMPH): protocol for a multicentric prospective observational study, Akshay Gupte, Chandrasekaran Padmapriyadarsini, Vidya Mave, Dileep Kadam, Nishi Suryavanshi, Shri Vijay Bala Yogendra Shivakumar, Rewa Kohli, Nikhil Gupte, Kannan Thiruvengadam, Anju Kagal, Sushant Meshram, Renu Bharadwaj, Sandhya Khadse, Geetha Ramachandran, Luke Elizabeth Hanna, Neeta Pradhan, NS Gomathy, Andrea DeLuca, Amita Gupta, Soumya Swaminathan, BMJ Open, Feb, 2016
- Modulation of dendritic cell and monocyte subsets in tuberculosis-diabetes co-morbidity upon standard tuberculosis treatment, Kumar NP, Moideen K, Sivakumar S, Menon PA, Viswanathan V, Kornfeld H, Babu S. Tuberculosis (Edinb). 2016 Dec; 101:191-200. PMID: 27865391; PMCID: PMC5127284.
- Effect of standard tuberculosis treatment on naive, memory and regulatory T-cell homeostasis in tuberculosis-diabetes co-morbidity, Kumar NP, Moideen K, Viswanathan V, Kornfeld H, Babu S. Immunology. 2016 Sep;149(1):87-97. PMID: 27289086; PMCID: PMC4981606.
- Glucose metabolism disorder is associated with pulmonary tuberculosis in individuals with respiratory symptoms from Brazil, Almeida-Junior JL, Gil-Santana L, Oliveira CA, Castro S, Cafezeiro AS, Daltro C, Netto EM, Kornfeld H, Andrade BB. PLoS One. 2016 Apr 14;11(4): e0153590. PMID: 27078026; PMCID: PMC4831681.
- High prevalence and heterogeneity of diabetes in patients with TB in South India: A report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study, Kornfeld H, West K, Kane K, Kumpatla S, Zacharias RR, Martinez-Balzano C, Li W, Viswanathan V. Chest. 2016 Jun;149(6): 1501-8. PMID: 26973015; PMCID: PMC4944775.
- Chandele A, Sewatanon J, Gunisetty S, Singla M, Onlamoon N, Akondy RS, Kissick HT, Nayak K, Reddy ES, Kalam H, Kumar D, Verma A, Panda H, Wang S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Medigeshi GR, Lodha R, Kabra S, Ahmed R, Murali- Krishna K. Characterization of Human CD8 T Cell Responses in Dengue Virus-Infected Patients from India. J Virol. 2016 Nov 28;90(24):11259-11278. Print 2016 Dec 15. PubMed PMID: 27707928; PubMed Central PMCID: PMC5126381.
- Raghavendhar BS, Ray P, Ratagiri VH, trial BS, Kabra SK, Lodha R. Evaluation of chikungunya virus infection in children from India during 2009-2010: A cross sectional observational study. J Med Virol. 2016 Jun;88(6):923-30. doi: 10.1002/jmv.24433. Epub 2015 Dec 15. PubMed PMID: 26581026.
- Anil Kr Verma, Anmol Chandele, Kaustuv Nayak, Murali-Krishna Kaja, Arockiasamy Arulandu, Pratima Ray. 2016. High yield expression and purification of Chikungunya virus E2 recombinant protein and its evaluation for serodiagnosis. Journal of Virol. Methods. 5: 003
- Kompithra RZ, Paul A, WordCleaner D, Babji S, Sarkar R,Mathew LG, Kang GImmunogenicity of a 3 dose and 5 dose oral humanrotavirus vaccine (RIX4414) schedule in south Indian infants. Vaccine 32Suppl 1:A129-A133. PMID: 25091666.
- Anil Kr Verma, Anmol Chandele, Murali-Krishna Kaja, Arockiasamy Arulandu, Pratima Ray. 2014. Cloning, expression and purification of Chikungunya virus E2 recombinant protein in E.coli. BMC Infectious Diseases. 14(Suppl 3):P65
- Paul A, Babji S, Sarkar R, Lazarus RP, Kang G. (2016) Rotavirus specific salivary and fecal IgA in Indian children and adults.Indian Pediatr;53601-606. PMID: 27508537.
Patent Applied:
IPA No. 202111003148: A novel method to evaluate the quality of antigen-specific T cells in infection and vaccination.
NCR BIOTECH SCIENCE CLUSTER-FARIDABAD
Regional Centre for Biotechnology (RCB), Translational Health Science and Technology Institute (THSTI), National Institute of Immunology (NII), National Institute of Plant Genome Research (NIPGR), and National Brain Research Centre (NBRC) have collaborated to jointly form the NCR Bio-Cluster, envisaged to support innovation, research, education, and entrepreneurship in varied areas of biotechnology through creative partnerships with Biotech & Pharmaceutical industry. While THSTI, NII, NIPGR, and NBRC are institutions primarily focused on research and development activities, RCB is an institution for academic training and research. The five partner institutions of the NCR Biotech Science Cluster together with participation from industry partners bring synergies in multi-disciplinary research, and operational efficiencies in the creation, management, and operation of the common facilities of the Cluster.
The Biotech Science Cluster has already achieved a unique blend of a variety of multiple biotech disciplines, in as much as, co-existence of RCB as a University, Research institutions as partner institutions connected with all the support systems and common facilities like Advanced Technology Platform Centre (ATPC), BSC BioNest Bio-incubator (BBB), Small Animal Facility (SAF), M.K. Bhan Auditorium, BSL-3 Facility, Biorepository and Hostels to support the innovation, translational research and commercialization endeavours in inter-disciplinary areas of biotechnology and life sciences.
During the FY 2020-21; the NCR Biotech Science Cluster has ensured completion of the following common facilities: a) Office of Connectivity Building, b) Biosafety Level-3 Facility, c) Vertical Extension of Hostel Building.
The Office of Connectivity has been conceptualized as the cluster office for the NCR Biotech Science Cluster and is responsible to establish a governance structure for the management and utilization of common facilities. It is working towards creating an innovative and efficient management structure, so that the advantage of having different institutions co-located in a cluster, with their respective competencies can be nurtured through systematic sharing of knowledge and resources. Office of Connectivity is acting as a hub for the whole cluster to focus on seamless connectivity for accomplishing best results by bringing together NCR Biotech Science Cluster stake-holders by facilitating, coordination, collaboration and sharing of efforts within the Cluster setting for various multidisciplinary collaborative research programs across the partner institutions along with the establishment and management of Common Facilities of the NCR Bio-cluster.
NCR Biotech Science Cluster

Office of Connectivity BSL-3 Facility

- Background of National Biopharma Mission
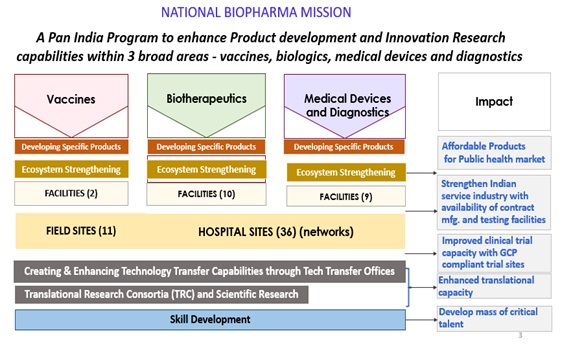
ItisanIndustry-Academia CollaborativeMissionofDepartment of Biotechnology(DBT),Govt of India foracceleratingdiscoveryresearch to earlydevelopment forBiopharmaceuticals. Approved by the Cabinet for a total cost US$ 250 million it is50%co-fundedbytheWorldBank.TheMission is implemented by BIRAC-thepublicsectorundertakingofDepartmentofBiotechnology, Ministry ofScience and Technology, Government of India, through adedicatedProgramManagementUnit(PMU)establishedatBIRAC.
Mission Mandate and Overview:
To enable and nurture an ecosystem for preparing India's technological and product development capabilities in biopharmaceuticals to a level that will be globally competitive over the next decade, and transform the health standards of India's population through affordable product development.
Thrust Areas of the Mission is depicted in figure below which includes 8 components in the area of vaccines, biotherapeutics and devices and Diagnostics
Thrust Areas of the Mission is depicted in figure below which includes 8 components in the area of vaccines, biotherapeutics and devices and Diagnostics
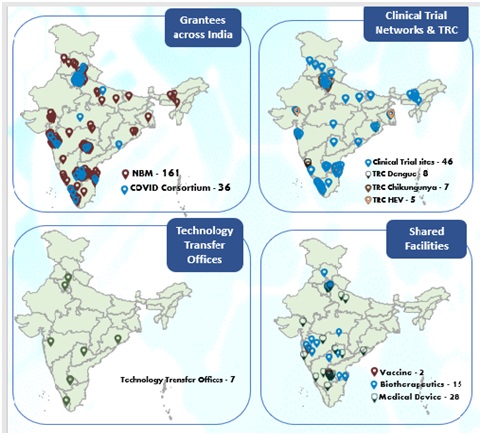
Overall Achievements:Since Mission has launched 34 Requests for Proposals and is supporting 197 grantees
Major Programs and Initiatives with achievements
Developmentofproductsandrelated ecosystem
Vaccines: Mission is supporting UniversalFlu vaccine,Cholera vaccine, Dengue(liveattenuatedandrecombinant),Chikungunya,Pneumococcal conjugate vaccine, and SARS-CoV-2vaccinecandidates.Thesevaccinecandidatesarecurrentlyunderdifferentstagesofdevelopment.
- 2 Vaccines (Non-Covid); Pneumococcal, Chikungunya in clinical trials; 1 Covid-19 vaccine advanced from POC to preclinical
Covid-19
- 2 Covid-19 Vaccines advanced to Clinical Trials (Bio E and Cadila, with further support to MCS received EUA
- , Insulin Glargine, Insulin Lispro, Ranibizumab, Aflibercept, Ustekinumab, and Herceptin are supported and are currently under different stages of development. The mission is also supporting indigenous development of clones for Ramucirumab, Golimumab and Factor VIII.
Biosimilars: The Mission supports therapeutic proteins and monoclonal antibodies (mAbs) that are currently under development by the industry with an aim to bring them closer to market. About 15mAbs which are not presently existing in Indian market and 03 Biosimilar clones, for diseases like cancer, diabetes, psoriatic arthritis, wet macular degeneration, Covid-19 are being supported. Indigenously developed Chimeric T-cell Receptor therapies and infrastructure for lentivirus manufacturing are supported.
- 2 Biosimilars advanced to Clinical trials
- 3 candidates Ustekinumab, Aflibercept, Herceptin completed Preclinical toxicity and DCGI approval awaited for Clinical trials
- Process optimization completed for continuous manufacturing of biosimilar Ranibizumab
Covid-19
- Pegylated interferon alpha-2b – Launched, in market
- Convalescent plasma therapy completed trials in 66 patients, the concentrated plasma is available and stocked
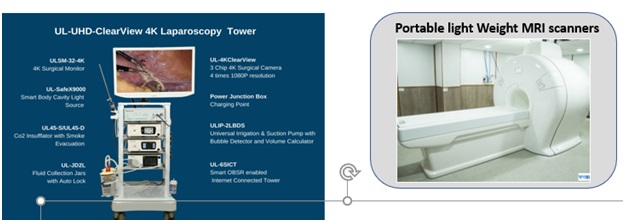
Medical Devices and Diagnostics:With a view to reduce import dependency, improve affordability and increase the innovation quotient, the Mission is supporting development of products in the areas as depicted below.
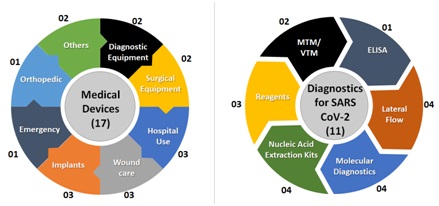
- 5 products in clinical validation stage
- 2 products developed, MRI medical grade camera, and portable MRI scanner
- 5 Covid Diagnostics products commercialized
Other Components Supported
Shared Facilities
For Vaccines:
The Good Clinical Lab Practices (GCLP) facilities are focusing on Pneumococcal (bacterial) assays (RVS KIMS) and serological assays for major viral diseases affecting the Indian subcontinent, Dengue and Chikungunya, (IRSHA, Pune).

For Biologics:
- The cGMP manufacturing facilities for process development and clinical lot of manufacturing of Biologics in cGMP certified facilities.

- The cell line repositories at National Centre for Cell Science (NCCS) and CSIR-IMTECH are under development. Activities are on progress for facility establishment and establishing MTAs with the international repositories for acquiring the cell line strains.
Out od 21 supported facilities, 15 are providing services to more than 150 industry, start-ups, MSMEs and academic sector.
Scientific Research:
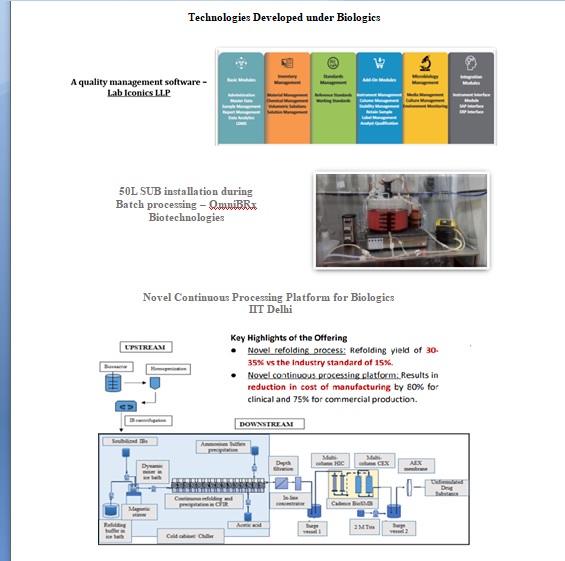
Promoting Indigenous manufacturing and novel Biologics:NBM has also funded several proposals promoting indigenous development of technologies for affordable biotherapeutics and novel therapies. The activities funded under this area include novel cell line development, resin preparation, disposable bioreactors, and IT platform.
Translational Research Consortia: The Mission is supporting five consortia, comprising 27 grantees
Goal of Translational Research Consortia (TRC) is to assemble, coordinate and develop translational research activity for building sustainable capacity in India towards Dengue, Chikungunya, Malaria and Hepatitis E virus (HEV). These are consortia comprising multidisciplinary units from various premier organizations of the country as depicted in map below.
Clinical Trial Network (CTN):Efforts are ongoing to augment the clinical trial capacity in the country for doing population based large Phase III clinical trials for COVID vaccines and Phase I to Phase III trials of drugs, biologics and devices. Eleven (11) field sites and 36 hospital sites. The personnel at these sites are trained for Good Clinical Practice and Good documentation practices and paperless data collection
Technology Transfer Offices:
Seven (07)TechnologyTransferOffices (TTOs)havebeenoperationalwith thesupportoftheMission.Over 200 technology showcasing and matchmaking events were held across these TTOs for technology licensing.A Practice manual for RTTOs developed which includes process and procedures along with all template documents for usage in technology licensing for the RTTOs.26 professionals supported under the Mission since then have beenrecognizedasRegisteredTechnologyTransferProfessionals(RTTPs)byATTP.
Skill Development:
The Mission supports trainings and workshops as per its mandate. Workshops in the areas ofclinical research, regulatory compliances, technology transfer, biopharmaceuticals and medicaldevices have been majorly supported. As on date, about 6678 participants have been trainedunderdifferenttrainingsandworkshopsundertheNationalBiopharmaMissionincluding 3038femaleparticipants.
Patents and Publications
| S. No. | Indicator Name | Actual Achieved |
| 1 | Number of international publications | 19 |
| 2 | Number of Patents | 20 |
New Technologies/Platforms Developed
Platforms Developed under Dengue Consortia
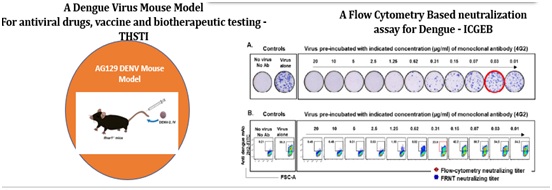
Technologies Developed for Vaccines

Technologies Developed under Medical Devices
Contacts Concerned Officer for more Information
| Programme Head | Dr. Alka Sharma, Scientist H |
|---|---|
| alka[at]dbt[dot]nic[dot]in | |
| Phone No. | 011-24363699 |
| Programme Officer | Phone No. | |
|---|---|---|
| Dr. Jyoti Malik Logani, Scientist F | jyoti[dot]logani[at]nic[dot]in | 011-24362329 |
| Dr. Varshneya Singh, Scientist D | varshneya[dot]singh[at]dbt[dot]nic[dot]in | 011-24360295 |
















