- GOVERNMENT OF INDIA
- MINISTRY OF SCIENCE & TECHNOLOGY
Key Statistics
 Biotech services Covid-19 78,22,344
Biotech services Covid-19 78,22,344  Ongoing Projects 1,484
Ongoing Projects 1,484  Projects Sanctioned 5,462
Projects Sanctioned 5,462  International Collaborative Projects 10
International Collaborative Projects 10  Scientists Supported 22,128
Scientists Supported 22,128  Research Personnel 48,502
Research Personnel 48,502  CTEP - Proposals Sanctioned
CTEP - Proposals Sanctioned 2,828
 Technology Generated 1,020
Technology Generated 1,020  Publications 22,708
Publications 22,708  Patents Filed 1,171
Patents Filed 1,171 

























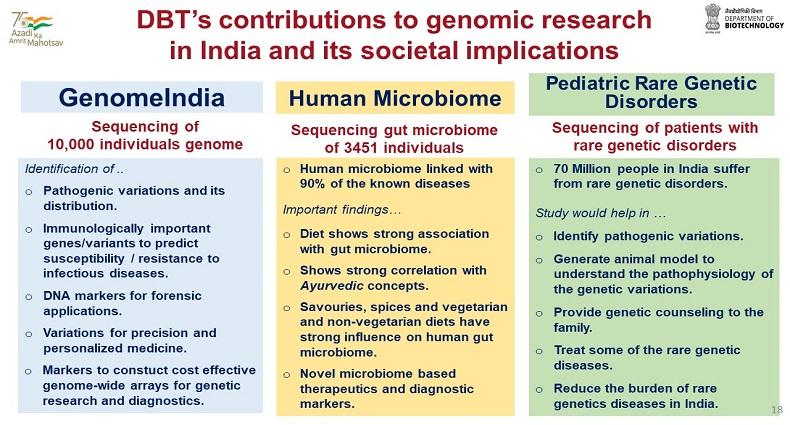
























 ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
, 
 ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
,  ,
, 














